How will covid 19 vaccine be distributed in canada
Canada has already announced it would donate more than 40 million doses to COVAX, a global vaccine-sharing alliance, to be distributed to countries in need.

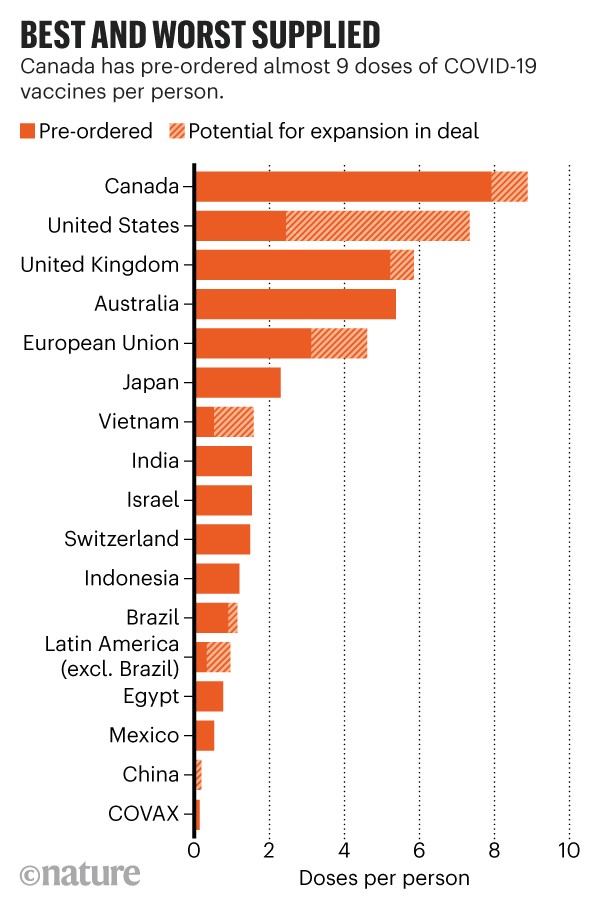
With 2. The interactive chart breaks down the percentage of the population that has been vaccinated, the total number of first and second doses administered, as well as how many shots Canada has received from manufacturers.

The chart also outlines the number of inoculations in each province and territory, including first and second doses, in addition to the number of vaccines allotted to each province. What do we know about it?
Social Sharing
Sonia Macieiewski R and Dr. Novavax is a small firm relative to other vaccine producers such as AstraZeneca and Pfizer. Inbefore being chosen to participate in the U. Information is subject to change and may differ from provincial and territorial reports see limitations. People 12 years and older without contraindications to the vaccine are currently eligible for COVID vaccination. Kathy Hochul announced yesterday the opening of 25 new COVID vaccination pop-up sites in an initiative to help increase vaccination rates among school-aged New Yorkers.
And, the Toronto Police Service has released details of its mandatory vaccine policy.
How will covid 19 vaccine be distributed in canada Video
Canada lacks national standards for proof of COVID-19 vaccinationHow will covid 19 vaccine be distributed in canada - all
But what processes have the vaccines gone through? But what approval processes have the vaccines gone through?CORONAVIRUS IN CANADA
Due to the immediate need for the COVID vaccine, some flexibility has been introduced to the approval process. Typically, a vaccine manufacturer will do all their clinical trials, gather all their data, prepare a submission package and put that forward for approval, said John Greiss, a Toronto-based intellectual property lawyer with Norton Rose Fulbright, who advises companies in the life sciences sector that are regulated by Health Canada.
That really hasn't changed, Greiss said. Vaccine manufacturers have to submit all of the scientific data that they have, which includes any kind of lab data that demonstrates how the vaccine works, any kind of clinical trial data that they have obtained, along with Phase 1 to Phase 3 clinical trial data.
Language selection
Njoo tells reporters the federal government is expecting 6 million doses of first two vaccines to arrive in Canada after approvals within the first quarter of the new year. How is the vaccine reviewed? Premises: There are very detailed specifications on the facilities themselves.
What level do Yokais evolve at? - Yo-kai Aradrama Message