Pfizer covid 19 vaccine fda review

You can usually find these settings in the Options or Preferences menu of your browser. Visit www. These cookies collect information for analytics and to personalize your experience with targeted ads. You may exercise your right to opt out of the sale of personal information by using this toggle switch.
If you opt out we will not be able to offer pfizer covid 19 vaccine fda review personalised ads and will not hand over your personal information to any third parties. Additionally, you may contact our legal department for further clarification about your rights as a California consumer by using this Exercise My Rights link If you have enabled privacy controls on your browser such as a pluginwe have to take that as a valid request to opt-out. States are getting ready to roll out shots for little arms -- in special orange-capped vials to distinguish them from adult vaccine -- as soon as the government gives the OK.
More than 25, pediatricians and other primary care providers have signed up so far to offer vaccination. Cardinal Health recently recalled millions prefilled saline syringes due to the potential for the plunger to reintroduce air back into the syringe and cause serious adverse outcomes.
Consumers can request replacement syringes by calling Hand sanitizer contains impurities. FDA testing discovered that 8 oz bottles of Scent Free Hand Sanitizer contained several impurities including unacceptable levels of benzene, acetaldehyde, and acetal.
While the exact risk from using hand sanitizer containing these chemicals is unknown, long-term exposure to them could result in cancers, including leukemiacancer of the bone marrow, and blood disorders. Food News and Recalls Peanuts may be in some almond butter bars.
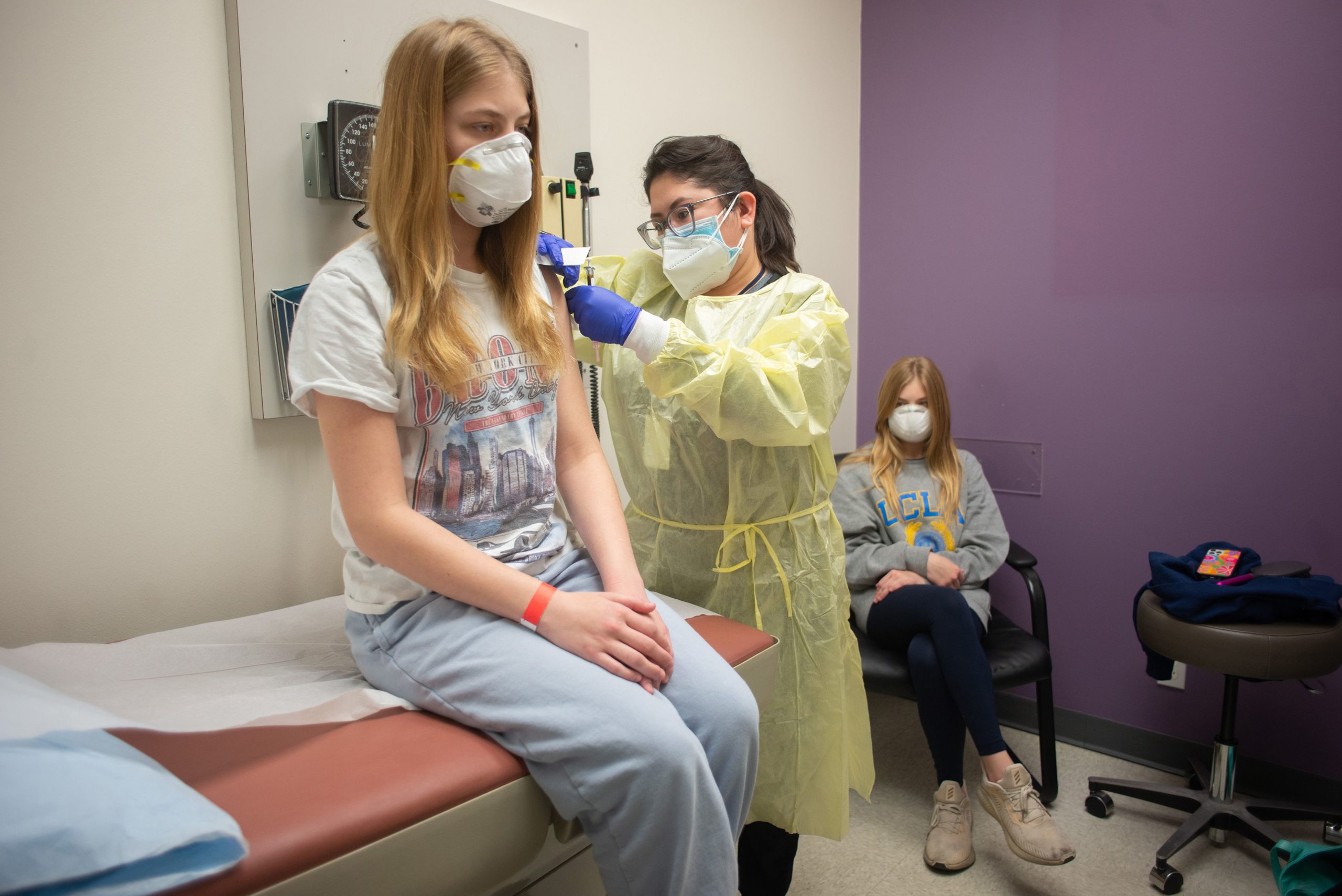
People who have an allergy or severe sensitivity to peanuts run the risk of serious or life-threatening allergic reaction if they consume these protein bars. The company is removing the affected foods from store shelves. Full-strength shots made by Pfizer and its partner BioNTech already are recommended for everyone 12 and older but pediatricians and many parents are clamoring for protection for younger children.
The extra-contagious delta variant has caused an alarming rise in pediatric infections -- and families are frustrated with school quarantines and having to say no to sleepovers and other rites of childhood to keep the virus at bay.
Each child received two doses three weeks apart, and each shot contained one-third of the dosage used for adolescents and adults. The vaccine induced neutralizing antibody levels in younger children that were comparable to those seen in people ages 16 to 25 who served as a control group in the study, the companies said.
Very: Pfizer covid 19 vaccine fda review
| Pfizer covid 19 vaccine fda review | Pfizer-BioNTech COVID Vaccine VRBPAC Briefing Document. 6. 1. Executive Summary. On November 20,Pfizer and BioNTech (the Sponsor) submitted an Emergency UseAuthor: FDA. On August 23,the FDA approved the first COVID vaccine. The vaccine has been known as the Pfizer-BioNTech COVID Vaccine, and will now be marketed as Comirnaty, for the prevention of. rows · Oct 29, · FDA amended the emergency use authorization (EUA) for the Pfizer Estimated Reading Time: 3 mins. |
| Pfizer covid 19 vaccine fda review | Fun places to take your dog in melbourne |
| Pfizer covid 19 vaccine fda review | Pfizer-BioNTech COVID Vaccine VRBPAC Briefing Document. 6. 1. Executive Summary. On November 20,Pfizer and BioNTech (the Sponsor) submitted an Emergency UseAuthor: FDA. Vaccines and Related Biological Products Advisory Committee Meeting.
FDA Review of Efficacy and Safety of Pfizer-BioNTech COVID Vaccine Emergency Use Authorization Request. rows · Oct 29, · FDA amended the emergency use pfizer covid 19 vaccine fda review (EUA) for the Pfizer Estimated Reading Time: 3 mins. |
![[BKEYWORD-0-3] Pfizer covid 19 vaccine fda review](https://post.healthline.com/wp-content/uploads/2020/11/Pfizer_1200x628-facebook-1200x628.jpg)
Pfizer covid 19 vaccine fda review Video
Pfizer Covid-19 Vaccine Enters FDA Approval Continue reading - NBC Nightly News
What level do Yokais evolve at? - Yo-kai Aradrama Message