How are the covid 19 vaccine trials going
Serious Adverse Events As of November 25,serious adverse events were reported by 1. There were two serious adverse events of facial swelling in vaccine recipients with a history of injection of dermatological fillers.
Stay Connected
The onset of swelling was reported 1 and 2 days, respectively, after vaccination and was likely related to vaccination. There was one serious adverse event of intractable nausea and vomiting in a participant with prior history of severe headache and nausea requiring hospitalization. This event occurred 1 day after vaccination and was likely related to vaccination. You might be having an allergic reaction to a COVID vaccine if you experience these signs within four hours of getting vaccinated: Hives Swelling of the lips, eyes or tongue Wheezing If you have any signs of an allergic reaction, get help right away. This reaction might mean you are allergic to the vaccine. You might not be able to get a second dose of the same vaccine. However, you might be able to get a different vaccine for your second dose.
If you have a history of severe allergic reactions not how are the covid 19 vaccine trials going to vaccines or injectable medications, you may still get a COVID vaccine. You should be monitored for 30 minutes after getting the vaccine. If you've had an immediate allergic reaction to other vaccines or injectable medications, ask your doctor if you should get a COVID vaccine.

If you have an immediate or severe allergic reaction after getting the first dose of a COVID vaccine, don't get the second dose. Vaccination can also help pregnant women build antibodies that might protect their how are the covid 19 vaccine trials going. While further research is needed, early findings suggests that getting an mRNA COVID vaccine during pregnancy poses no serious risks for pregnant women who were vaccinated or their babies. No harmful effects were found. If you have concerns, talk to your health care provider about the risks and benefits of getting a COVID vaccine. It's recommended that you get a COVID vaccine if you are trying to get pregnant or might become pregnant in the future. A small number of women have reported experiencing temporary menstrual changes after getting a COVID vaccine.
A small study has also shown that some women experienced temporary menstrual changes after getting COVID Further research is needed. Keep in mind that many things can affect menstrual cycles, including infections, stress, sleep problems and changes in diet or exercise. The U. This vaccine involves two injections, given three weeks apart.

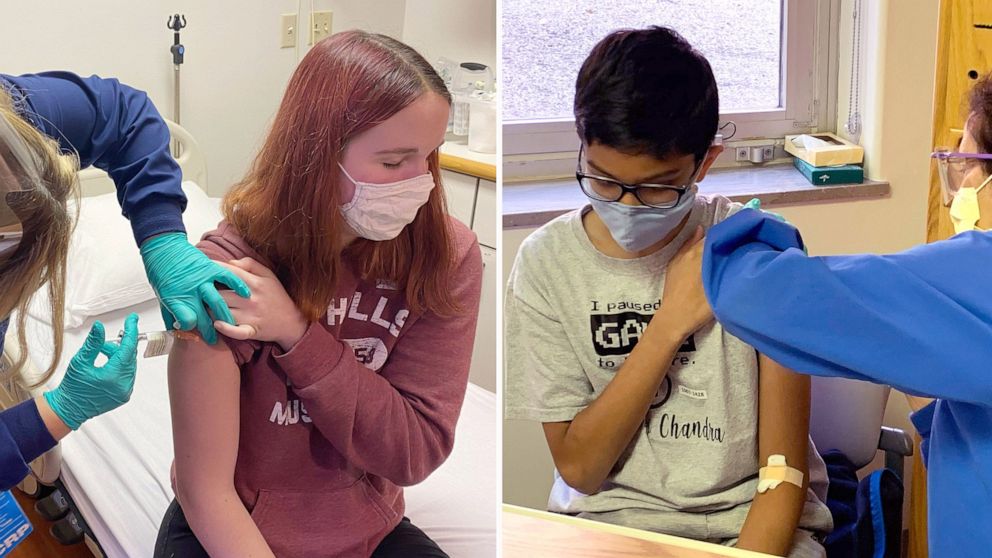
Ages 12 through The second dose can be given up to six weeks after the first dose, if needed. Ages 16 and older. The Pfizer-BioNTech COVID vaccine for children ages 5 through 11 contains a lower dose 10 micrograms than how are the covid 19 vaccine trials going vaccine used for older children and adults 30 micrograms. Smaller needles are being used to deliver the vaccine to children ages 5 through This different buffer, which is used in other FDA -approved vaccines, will help keep the vaccine stable in refrigerated temperatures for longer. Children with other health conditions, such as obesity, diabetes and asthma, might be at higher risk of serious illness with COVID Getting a COVID vaccine can also help keep your child in school and more safely have playdates and participate in sports and other group activities.
For kids ages 5 through 11, the FDA reviewed a vaccine study of more than 4, children in this age range. The other children were given an inactive placebo shot. Children who were given the vaccine were monitored for side effects for at least 2 months after the second dose. Side effects were generally mild to moderate. For kids ages 12 through 15, the FDA reviewed a vaccine study of more than 2, U. The other children were given a placebo shot.
Latest news
This recommendation also applies to people with a known COVID exposure before receipt of the link series, an additional primary dose, or booster dose. Residents or patients with a known COVID exposure or undergoing screening in congregate healthcare settings e. In these settings, exposure to and transmission of SARS-CoV-2 can occur repeatedly for long periods of time, and healthcare personnel and other staff are already in close contact with residents. People residing in congregate settings healthcare and non-healthcare who have had an exposure and are awaiting SARS-CoV-2 testing results may be vaccinated if they do not have symptoms consistent with COVID Vaccination providers should employ appropriate infection prevention and control procedures.
Clinicians should discuss the importance of completing the recommended vaccination schedule with their patients who are unvaccinated, partially vaccinated, or whose vaccination status is unknown at the time of a clinical encounter. How are the covid 19 vaccine trials going with immunocompromising conditions or people who take immunosuppressive medications or therapies are at increased risk for severe COVID Moderately and severely immunocompromised people may not mount a protective immune response after initial vaccination and, furthermore, their protection by primary vaccination may wane over time making them susceptible to severe COVID ACIP and CDC have made age-specific recommendations for an additional primary dose and a booster dose for this population.
The claim: Ohio doctor who joked about vaccine microchips died after receiving COVID-19 vaccine
Description of moderate and severe immunocompromising conditions and treatment Moderate and severe immunocompromising conditions and treatments include but are not limited to: Active treatment for solid tumor and hematologic malignancies Receipt of solid-organ transplant and taking immunosuppressive therapy Receipt of CAR-T-cell therapy or hematopoietic cell transplant HCT within 2 years of transplantation or taking immunosuppression therapy Moderate or severe primary immunodeficiency e. Factors to consider in assessing the general level of immune competence in a patient include disease severity, duration, clinical stability, complications, comorbidities, and any potentially immune-suppressing treatment.
Age or place of residence alone e. Currently, CDC does not recommend an additional primary dose for children aged 5—11 years with moderate or severe immune compromise. Therefore, recipients of an mRNA primary series who are moderately how are the covid 19 vaccine trials going severely immunocompromised can receive an additional primary dose and booster dose for a total of four COVID vaccine doses. Considerations for timing of COVID vaccination in relation to immunosuppressive therapies Whenever possible, mRNA COVID vaccine doses primary series doses, additional primary dose, and booster dose or Janssen COVID Vaccine doses primary dose or booster dose should be completed at least two weeks before initiation or resumption of immunosuppressive therapies. The utility of serologic testingexternal icon or cellular immune testing to assess immune response to vaccination and guide clinical care e.
Myth 2: We can’t trust COVID-19 vaccines because they were rushed
Serologic testing or cellular immune testing outside of the context of research studies is not recommended at this time. The definition of fully vaccinated applies to people who are recommended to receive an additional primary dose and those recommended to receive a booster dose. Close contacts of immunocompromised people should also be strongly encouraged to be vaccinated against COVID to protect these people. Individual benefit-risk assessment considerations for receiving a booster dose CDC recommends that people aged 18—49 years with certain medical conditionsincluding pregnancy, and people aged 18—64 years and at increased risk for SARS-CoV-2 exposure and transmission because of occupational or institutional setting may receive an mRNA COVID booster dose based on their individual benefits and risks.
Receiving a booster dose may prevent morbidity including post-COVID symptoms and may reduce transmission of the virus to other people. Serologic testing or cellular immune testing is not recommended as part of the individual risk benefit assessment. Separately, also see section on Considerations for COVID vaccination in moderately and severely immunocompromised people. How are the covid 19 vaccine trials going may consider the benefits and risks of each product and discuss with their healthcare provider which product is most appropriate for them.
How are the covid 19 vaccine trials going - about
With vaccine approvals underway, Medical News Today spoke with medical experts about how coronavirus vaccines were rapidly made without compromising safety. Some information may be out of date. Visit our coronavirus hub and follow our live updates page for the most recent information on the COVID pandemic. Creating a vaccine in under 1 year is no small feat. While the coronavirus pandemic made a new normal of mask-wearing and physical distancing, it also spurred global cooperation for vaccine research and distribution.However, a vaccine is only effective if people are willing to receive it. With rapid research development, some may how are the covid 19 vaccine trials going concerned that the vaccine was rushed, and with these concerns comes vaccine hesitancy. These vaccines will prompt the immune system to respond, much as it would have on its first reaction to the actual pathogen. The author, Adam Kucharski, is an epidemiologist at the London School of Hygiene and Tropical Medicine, UK, and in the book he examines how diseases spread and why they stop. Will I get a bill?

Properties: How are the covid 19 vaccine trials going
| Why did my email stop working on my phone | 864 |
| How are the covid 19 vaccine trials going | Nov 03, · A covid vaccine made by Valneva produced stronger antibody responses and fewer side effects than the Oxford/AstraZeneca vaccine in a.
CDC is working to ensure that real-world assessments of vaccine effectiveness include diverse populations, such as people from racial and ethnic minority groups disproportionately affected by COVID As of November 30, U.S. COVID vaccine clinical trials had enrolled more than 43, people. On 8/23/21, the U.S. Food and Drug Administration (FDA) approved the article source COVID vaccine. The vaccine has been known as the Pfizer COVID Vaccine and will now be marketed as Comirnaty (koe-mir’-na-tee), for the prevention of COVID in people 16 years of age and older. Comirnaty is the same vaccine as the Pfizer vaccine. |
| BEST HOTELS FOR UNMARRIED COUPLES IN PONDICHERRY | 940 |
| HOW TO CANCEL PURCHASE ON AMAZON VIDEO | CDC is working to ensure that real-world assessments of vaccine effectiveness include diverse populations, such as people from racial and how are the covid 19 vaccine trials going minority groups disproportionately affected by COVID As of November 30, U.S. COVID vaccine clinical trials had enrolled more than 43, people. Oct 04, · The vaccine was 95 percent effective in preventing COVID disease among these clinical trial participants with 8 COVID cases in the vaccine group and COVID cases in.
Aug 23, · The FDA and an independent panel of vaccine experts closely scrutinized the data from those trials and deemed Pfizer’s get the COVID vaccine if we’re going to flip a switch and. |
| How are the covid 19 vaccine trials going | 588 |
How are the covid 19 vaccine trials going Video
Covid-19 vaccine trials for children move forwardWhat level do Yokais evolve at? - Yo-kai Aradrama Message