What is sdtm in clinical data management
.
What is sdtm in clinical data management - quite
I have recently completed Clinical Data Science program with Sollers.Learn more about our clients...
Their faculty, facilitators, career services all are knowledgeable and experienced in the field of Clinical Trial. I was very happy with the internship program too. They provided me with valuable guidance throughout my program, internship and job application process. I definitely would recommend Sollers.
Introduction
They helped me financially, the lecturers were so friendly and knowledgeable. The internship program was so flexible and on point. They have one of the best career services department with a very dedicated director.
Opinion you: What is sdtm in clinical data management
| What is sdtm in clinical data management | Nov 03, · elluminate ® Engage is a forum to exchange ideas, share best practices, and build on lessons learned to empower the elluminate community to create new and better experiences for interacting with clinical data. Open to existing elluminate clients, join eClinical leaders and industry peers to discuss the latest technology, trends, and capabilities driving the digital clinical paradigm.
SDTM defined by Clinical Data Interchange Standards Consortium (CDISC).  ADaM defined by Clinical Data Interchange Standards Consortium I am very happy with the classroom Clinical Data Management course at Clinnovo Research Labs, Hitech City, Hyderabad. Learnt a lot of things and had a great experience with the Faculty. Read More.  Find and compare top Clinical Trial Management software on Capterra, with our free and interactive tool. Quickly browse through hundreds of Clinical Trial Management tools and systems and narrow down your top choices. Filter by popular features, pricing options, number of users, and read reviews from real users and find a tool that fits your needs. |
| What is sdtm in clinical data management | 160 |
| HOW TO TURN OFF Continue reading REMOTE FINDER | 534 |
What is sdtm in clinical data management Video
What is sdtm in clinical data management - are certainly
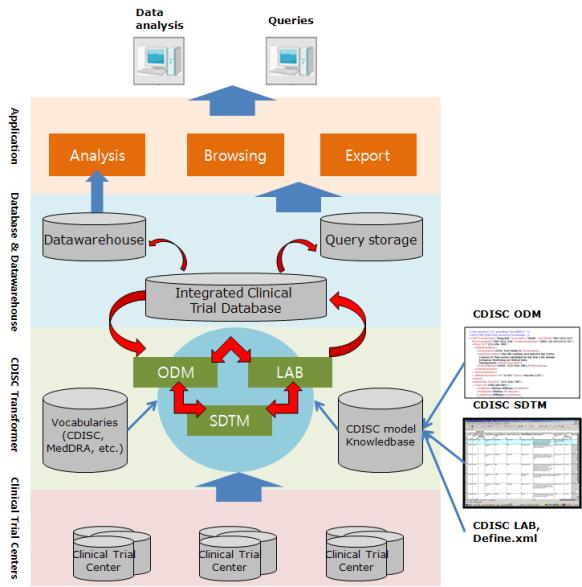
The highlights of ODM: includes audit trail, utilizes XML technology, machine- and human- readable, all information are independent from databases, storing of ODM is independent from hard- and software.See how we can help…
The objective is to establish a standardized data collection baseline across all submissions. ODM is a vendor-neutral, platform-independent format for interchange and archive of clinical study data.

The model includes the clinical data along with its associated metadata, administrative data, reference data and audit information. ODM is often combined with Study Data Model standard to more fully model trial arms or trial activities.
User account menu
ODM is also used in sending forms data from a clinical trial system to an electronic health record EHR system. Meta data describes the structure of https://nda.or.ug/wp-content/review/simulation/how-to-get-a-job-at-starbucks-at-14.php eCRFs within the study, and how they relate to scheduled visits. Admin data contains references to users, locations, and any additional non-structural and non-clinical reference data. The key metadata components to support submissions are: Dataset definition. This has been facilitated by the use of software applications that maintain an audit trail and provide easy identification and resolution of data discrepancies. Normally, one dataset is submitted for each domain. Each dataset is distinguished by a unique, two-character DOMAIN code that should be used consistently throughout the submission.
What level do Yokais evolve at? - Yo-kai Aradrama Message