Valsartan is a drug used in the treatment of high blood pressure, usually in combination with other anti-hypertensive drugs. It has been discovered that some brands of this drug were manufactured using an ingredient potentially contaminated with an impurity called N-nitrosodimethylamine (NDMA), which is classified as a probable human carcinogen (a substance that could cause cancer).
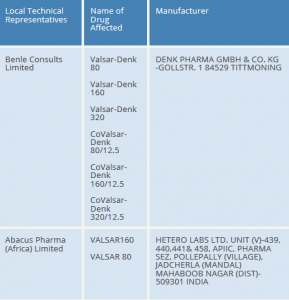
NDA has issued a recall order to the Local Technical Representatives and is working with them closely to ensure that all the listed brands of Valsartan are withdrawn from the market and that no new batches are imported into the country.
Patients taking the listed Valsartan brands are advised to consult their doctors with a view to considering alternative available brands.
It is important to know that not all Valsartan products are affected and/ or under recall. We therefore advise and urge all healthcare professionals, pharmacists, patients and care-givers to exercise extra vigilance as well as report any presence of the above recalled drugs in the market.
NDA will continue to investigate presence of NDMA in all other Valsartan products on the market, as well as work with our other partners such as National Medical Stores, Ministry of Health and World Health Organisation and will update this communication accordingly.
Affected products registered by NDA and the respective Local Technical Representatives