NDA’s Quality Control Laboratory is prequalified by World Health Organization and, has ISO/IEC 17025:2017 international standard accreditation by ANAB, United States of America. The Laboratory analyzes different categories of medicines, medical devices and public health care products.


Inspectorate and Enforcement Directorate ensures that medicines imported into the country are from approved manufacturing sources, and properly handled in a distribution supply chain to maintain quality and traceability.
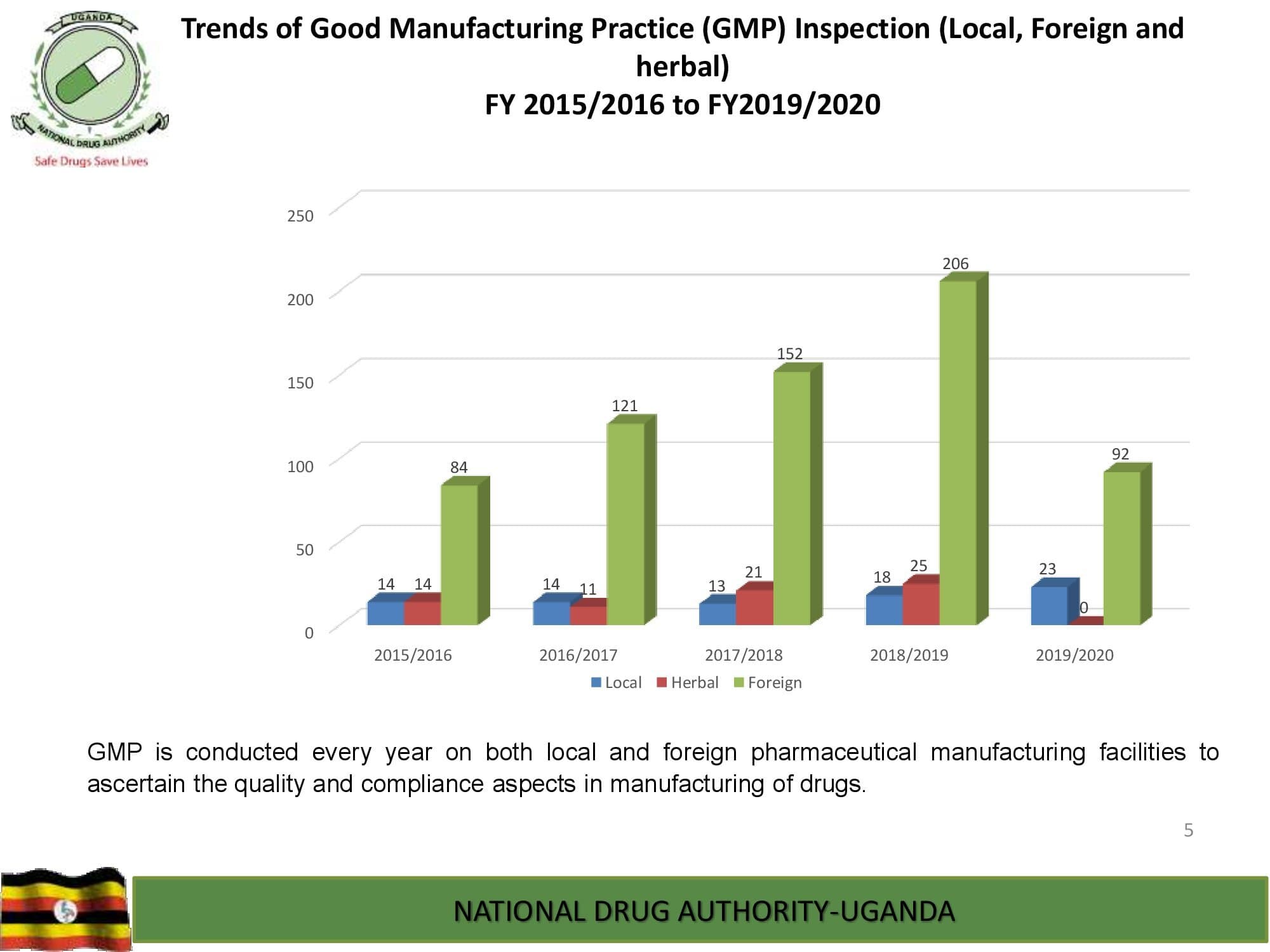
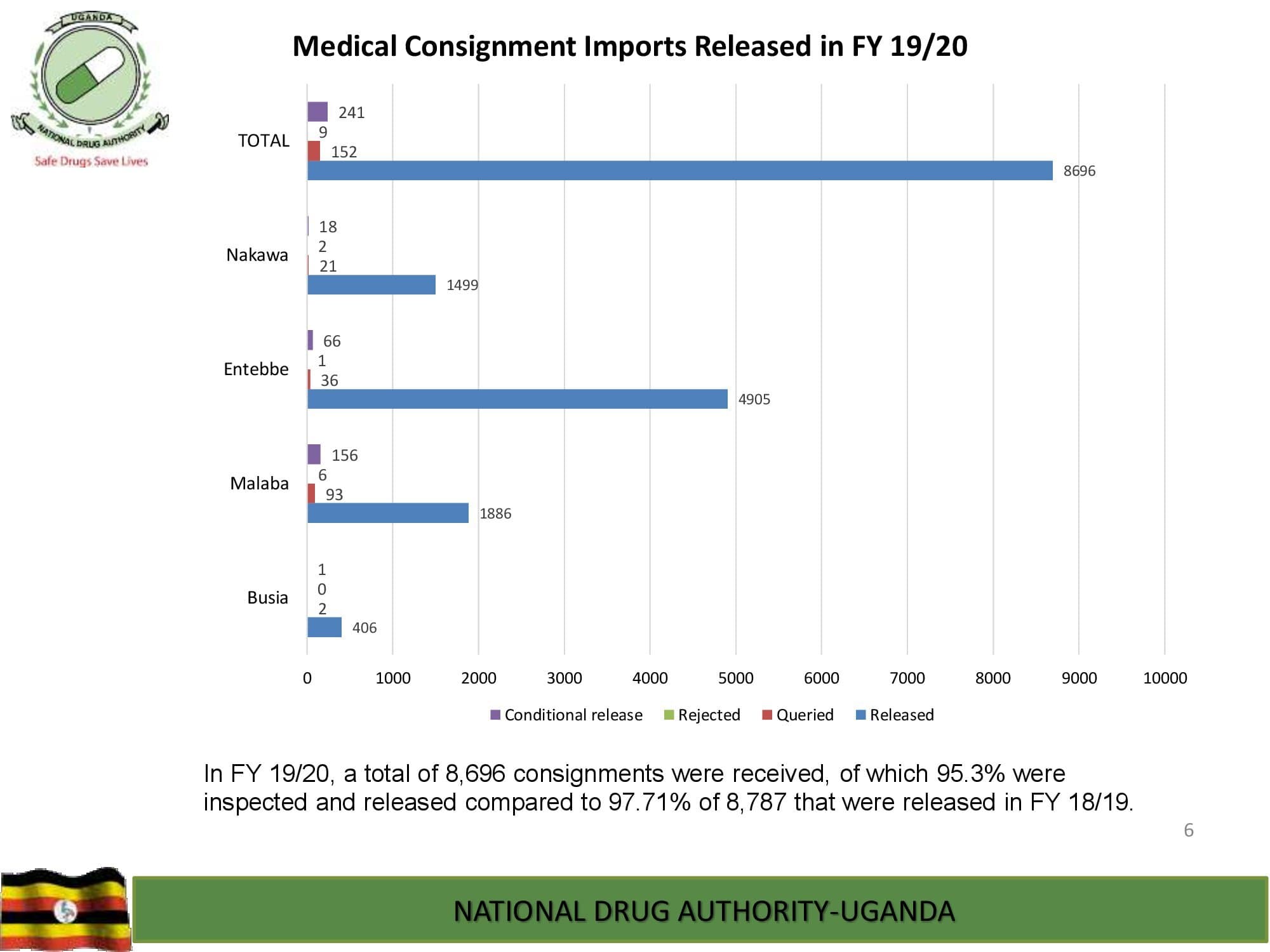
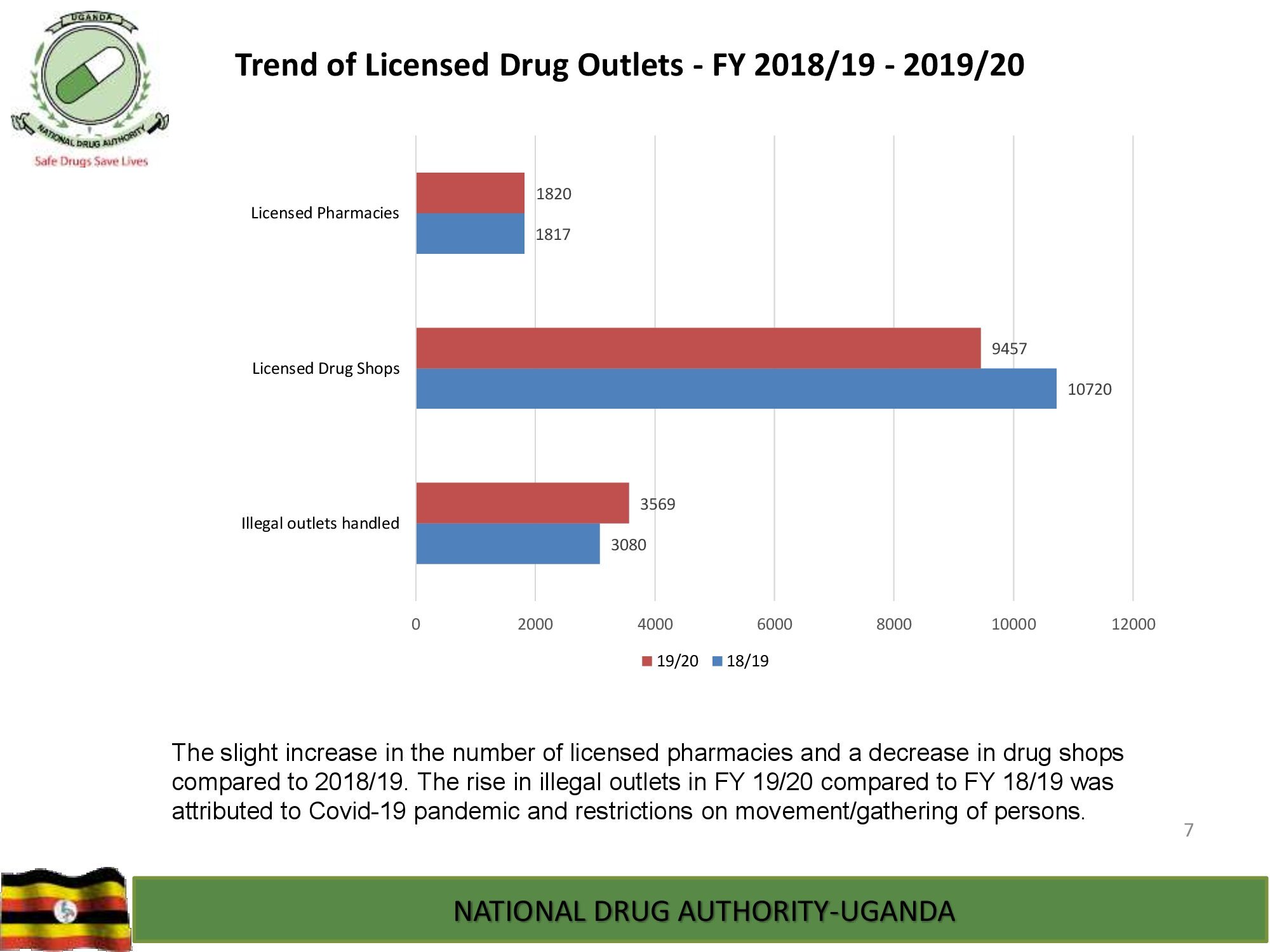
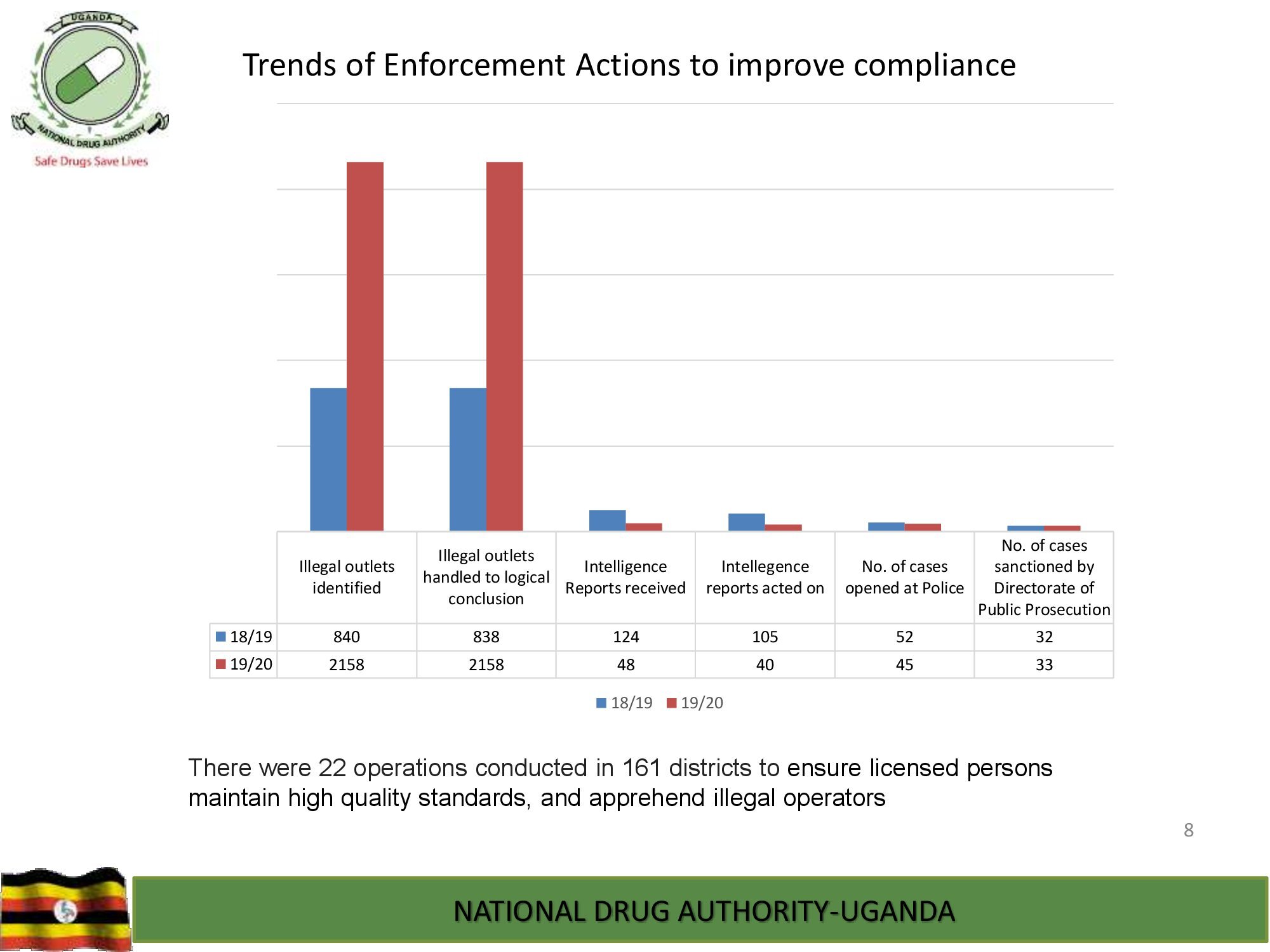

NDA’s Quality Control Laboratory is prequalified by World Health Organization and, has ISO/IEC 17025:2017 international standard accreditation by ANAB, United States of America. The Laboratory analyzes different categories of medicines, medical devices and public health care products
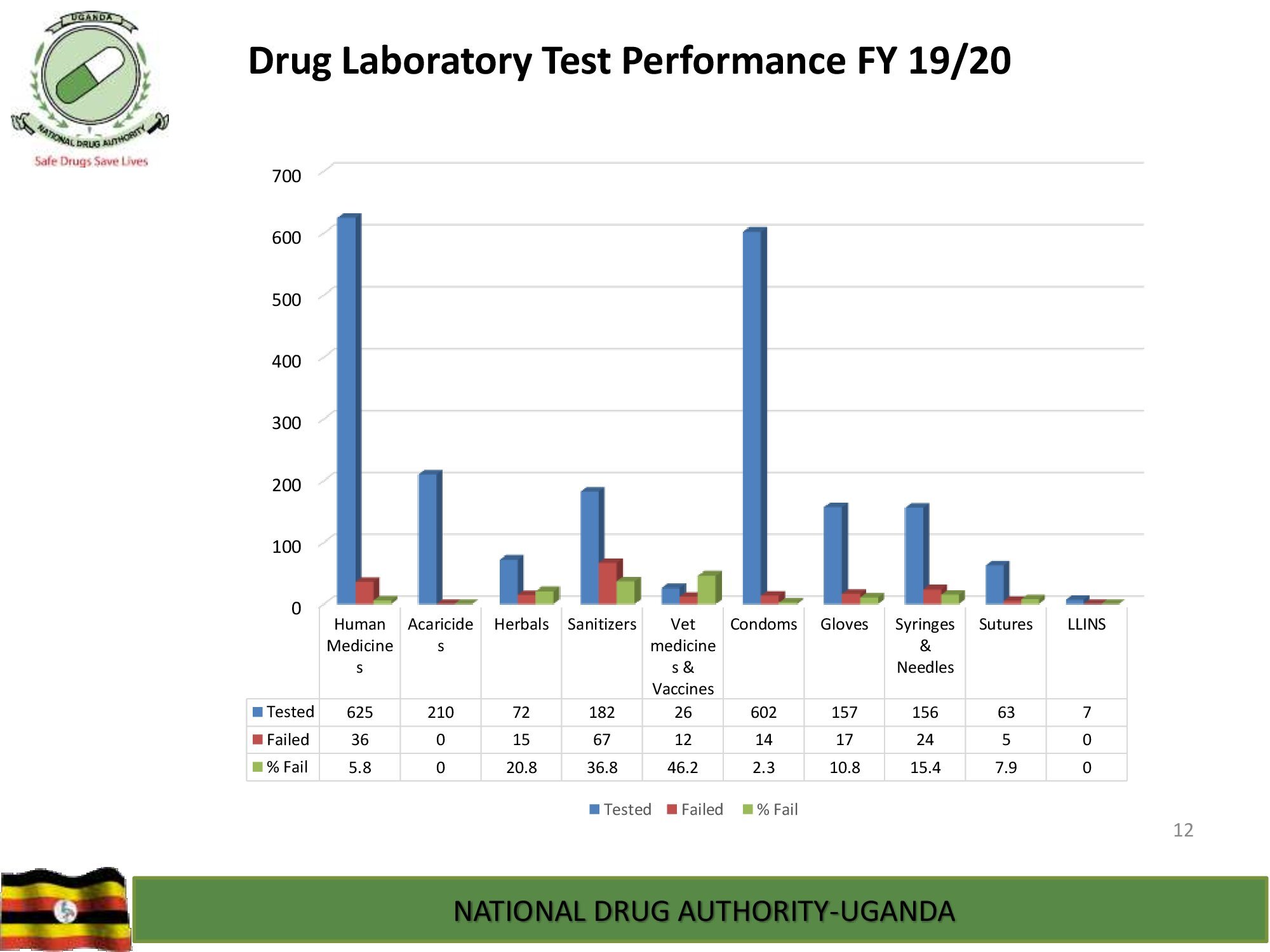
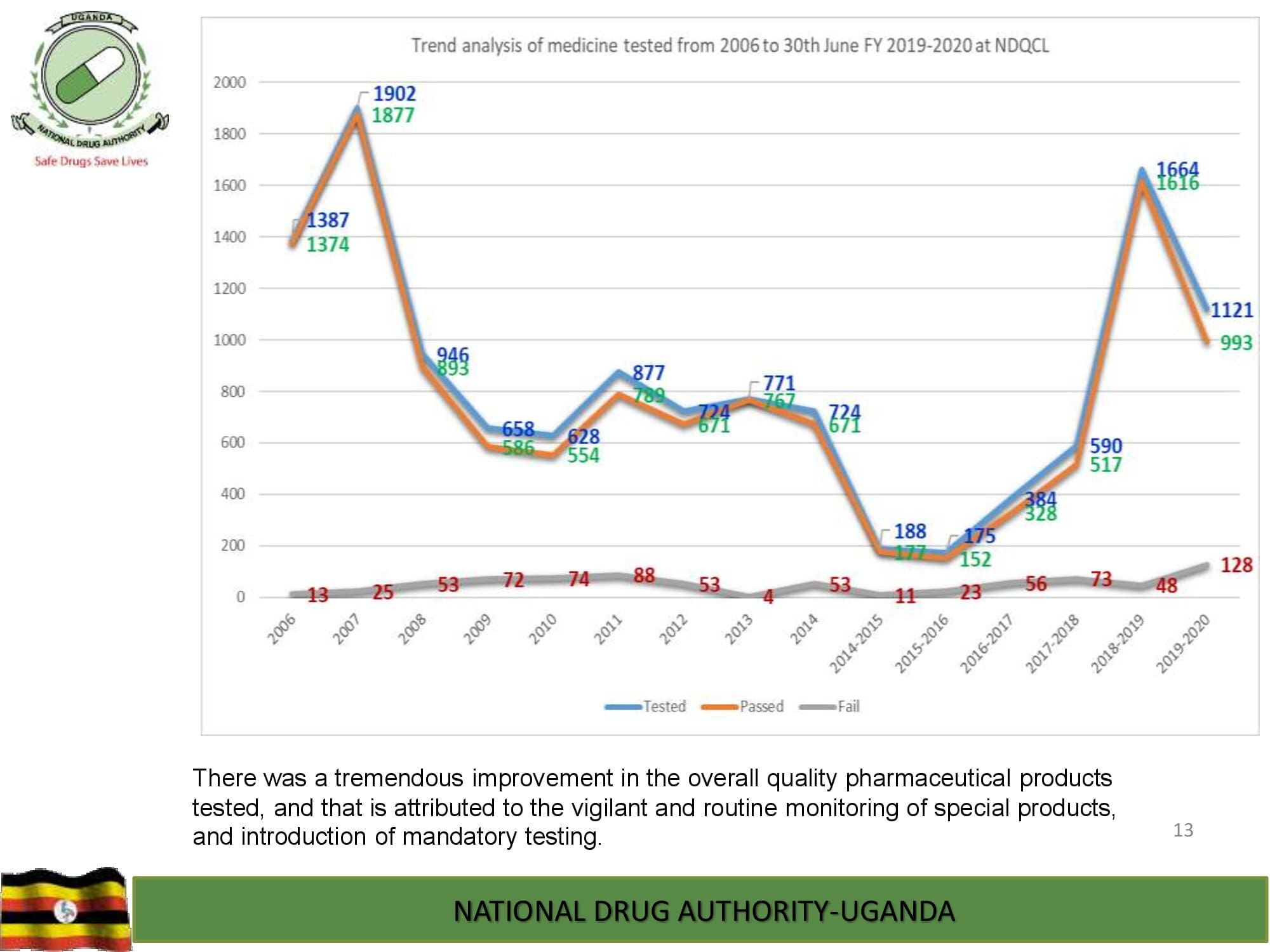
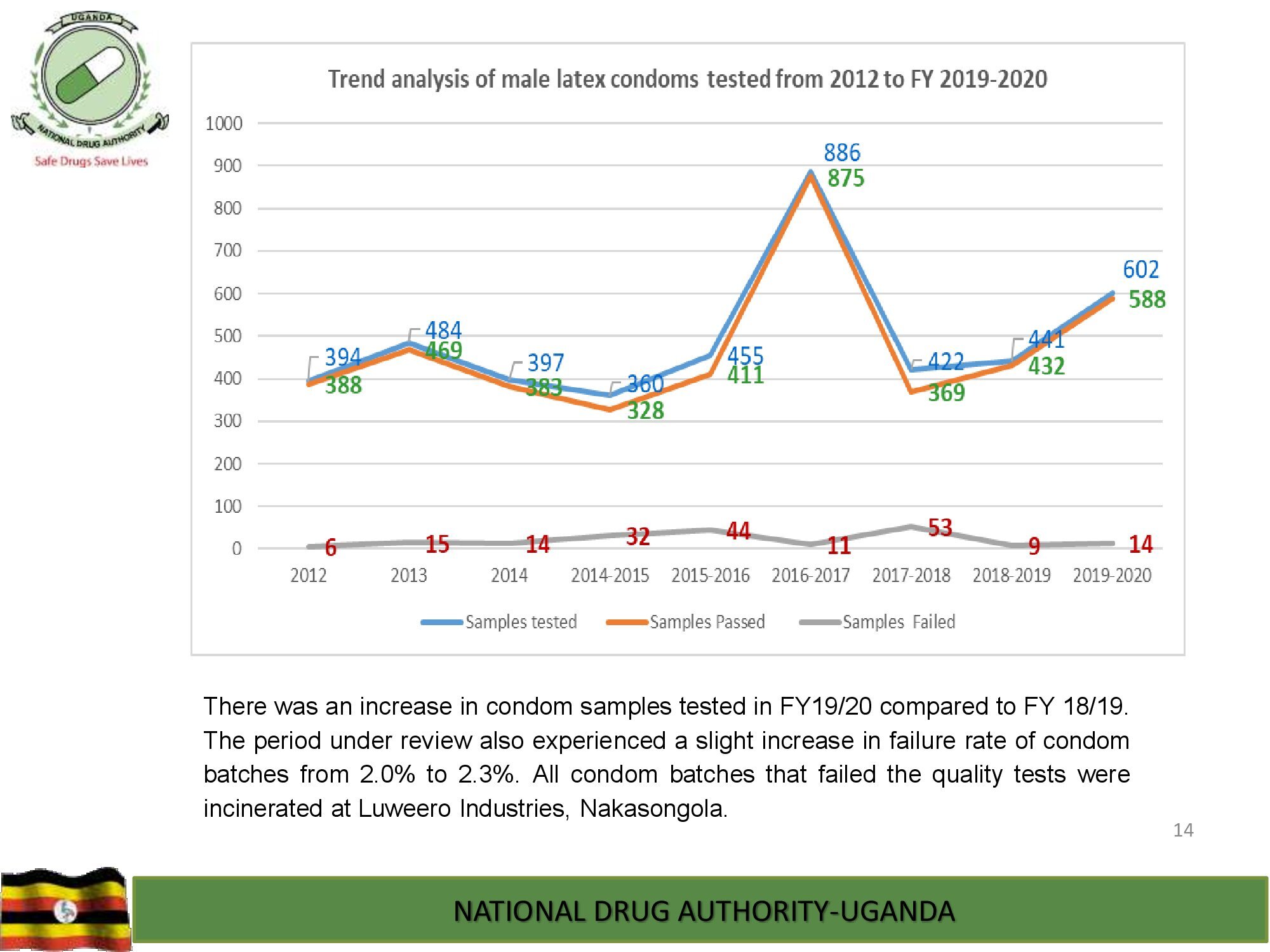
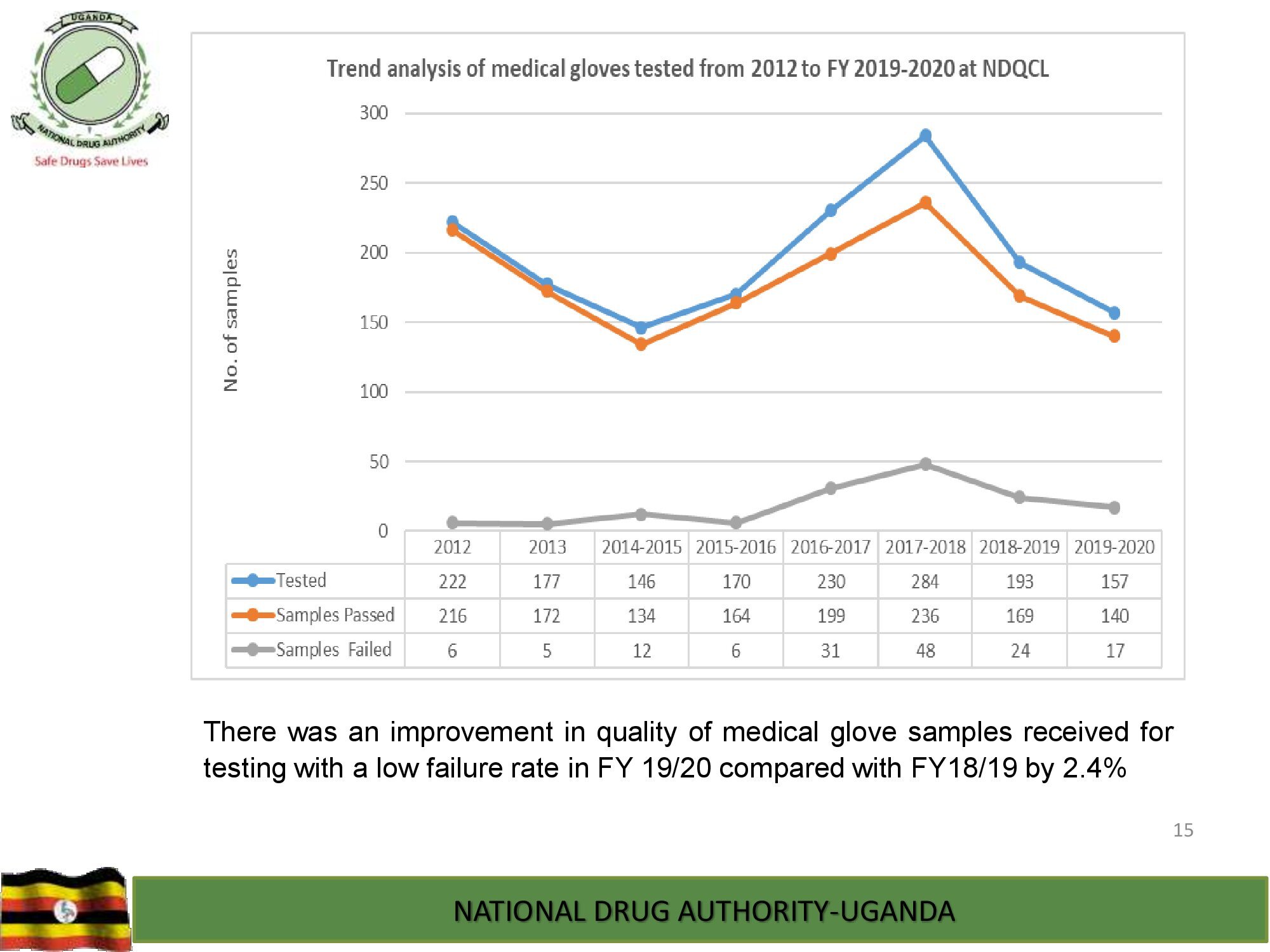
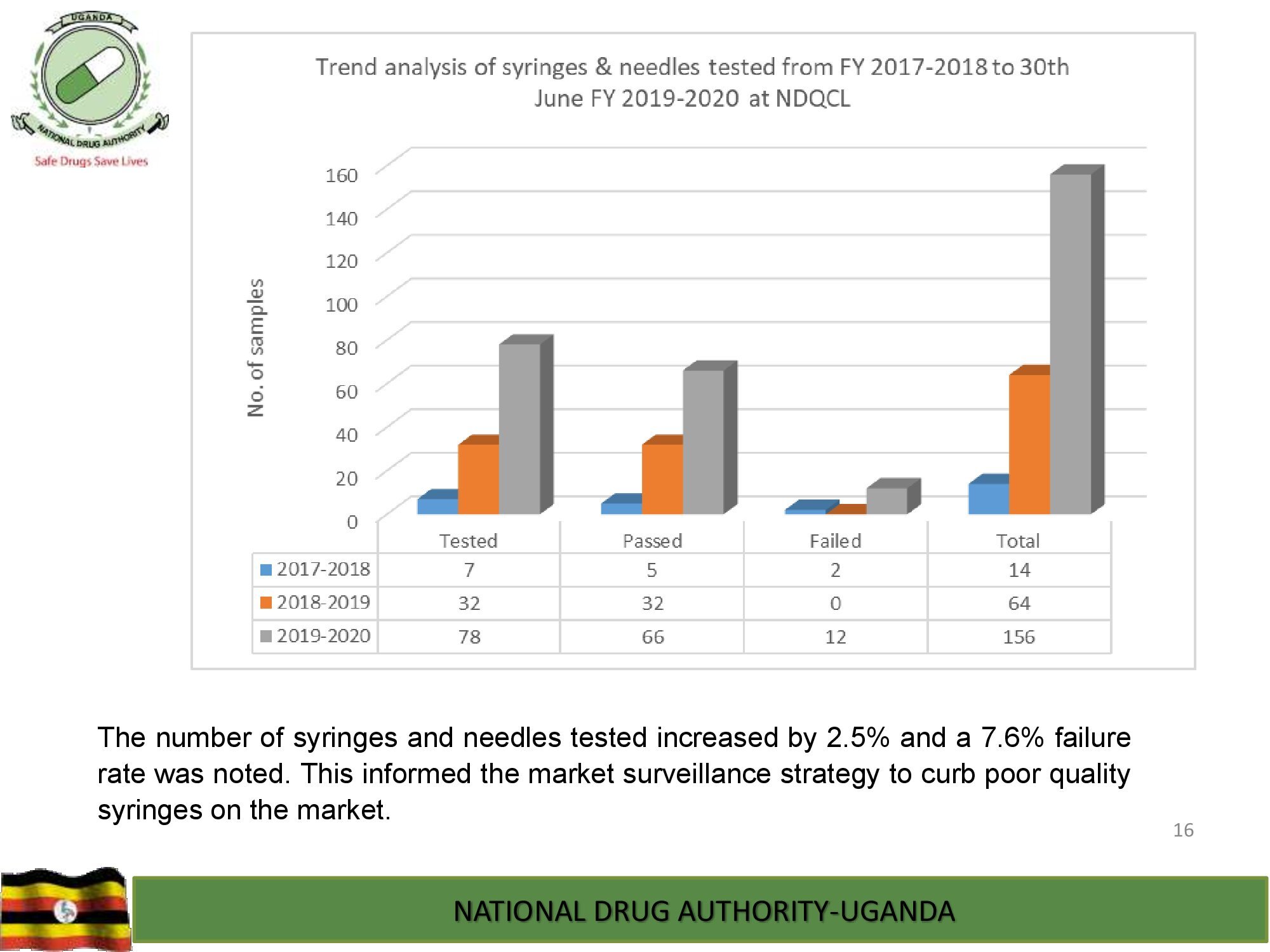
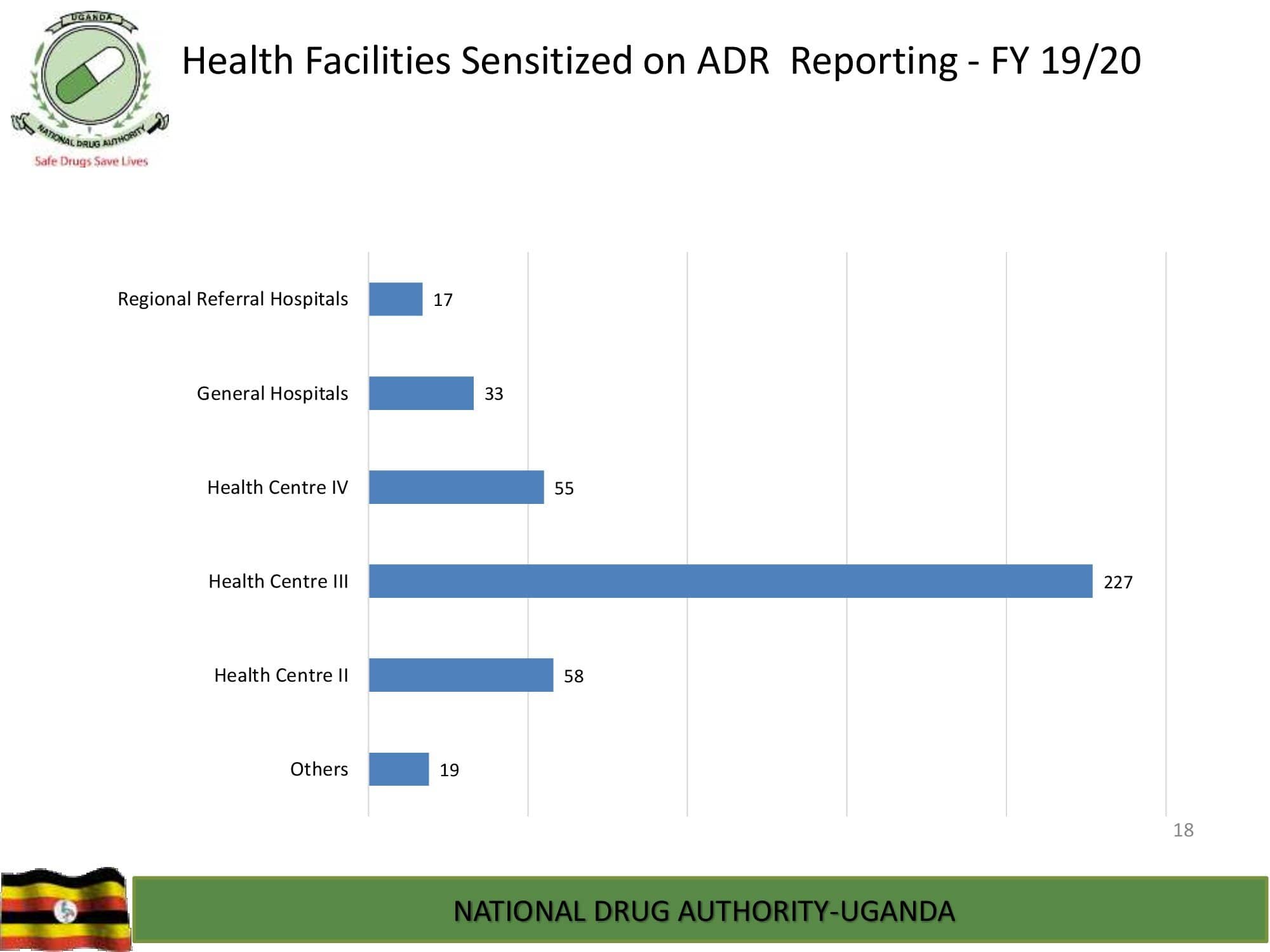
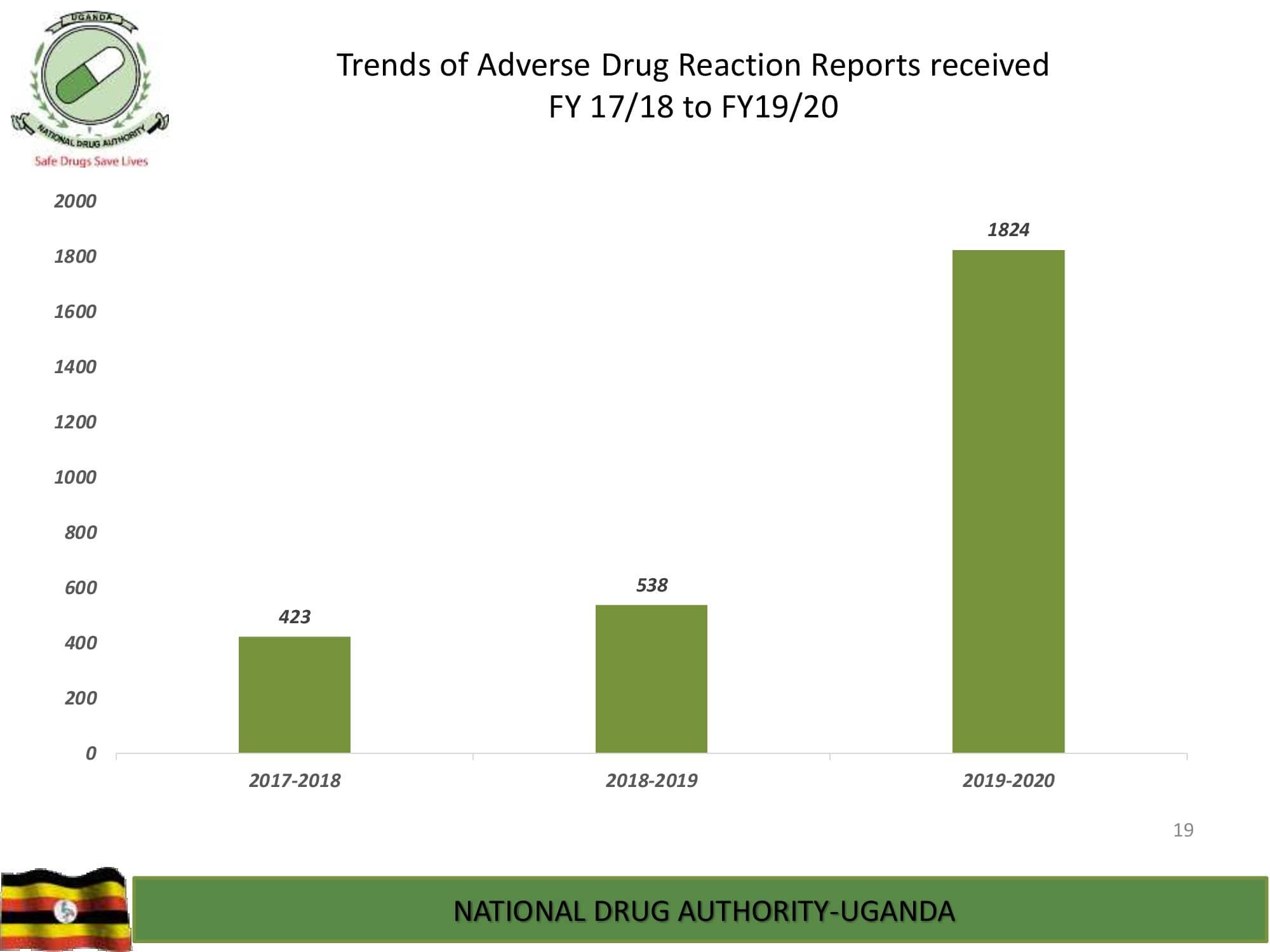
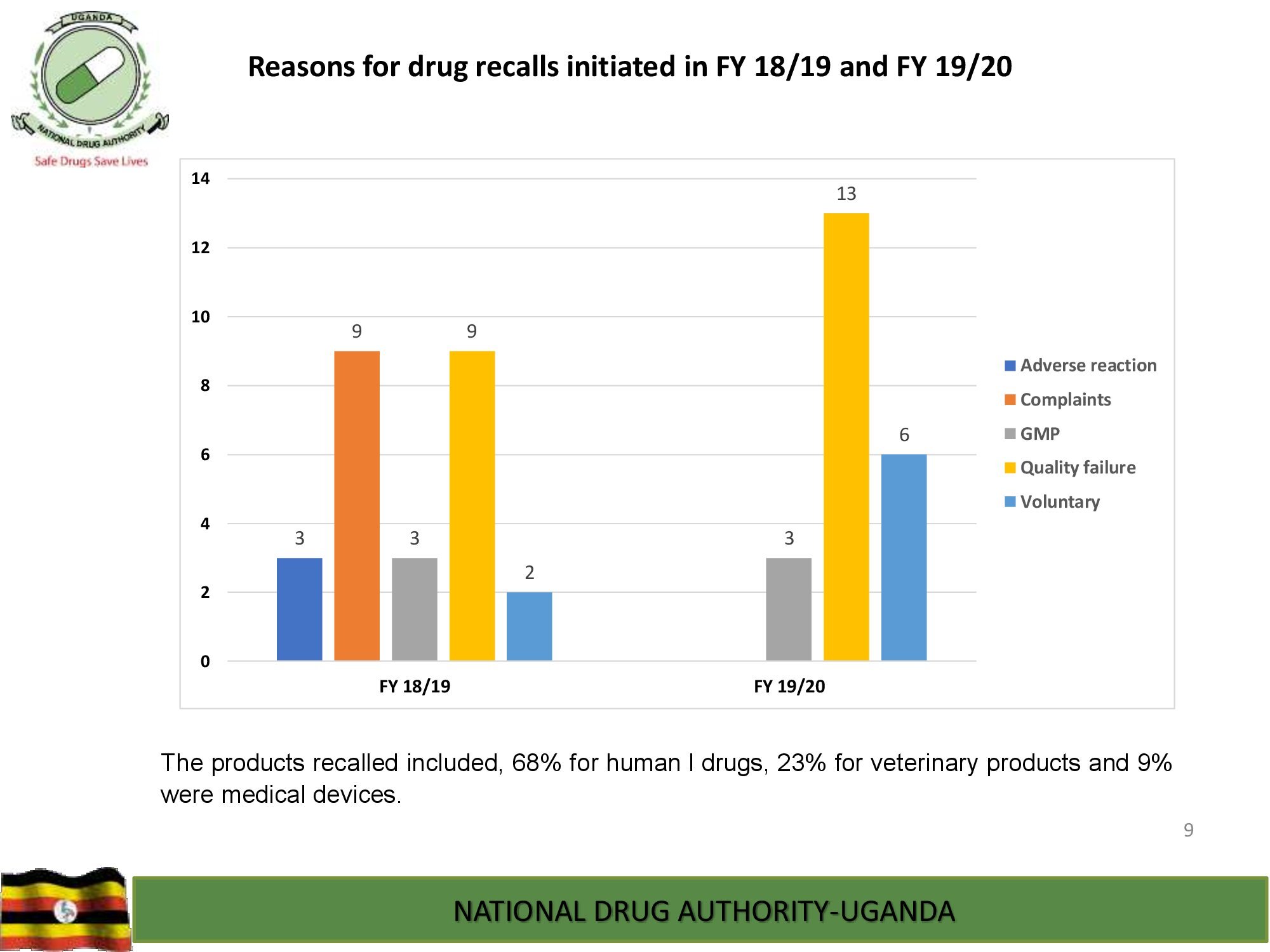
Product Safety Directorate is responsible for is responsible for promoting patient safety by monitoring adverse drug events to improve health outcomes.